Introduction
From 1 June 2019, faculty research that qualifies for an Exemption may be reviewed and approved by a Departmental Ethics Review Committee (DERC). This applies to social, behavioural and educational research (SBER) that is of minimal risk, but excludes any human biomedical research (HBR) that falls within section 3 of the Human Biomedical Research Act (HBRA).
All student and faculty research involving humans as research participants must be subject to ethics review by either the NUS Institutional Board (NUS-IRB) or the Department. The Department Ethics Review Committee (DERC) was set up in May 2017 as a representative of the Head of Department to review student research, and as of July 2019, faculty research as well. They will determine whether the research can be exempted or expedited (minimal risk projects) or referred to the NUS-IRB for review based on the risk to the participants. However, all human biomedical research as defined by section 3 of the Human Biomedical Research Act (HBRA) 2015 must be referred to the NUS-IRB for review.
DERC Members
Human Biomedical Research (HBR) and research that cannot be approved by DERC must be submitted to NUS-IRB via iRIMS.
Department of Pharmacy DERC was set up to expedite the approval process for educational research that qualifies for exemption. Educational research that qualifies for an Exemption may be reviewed and approved by the DERC. This applies to social, behavioural and educational research (SBER) that is of minimal risk, but excludes any human biomedical research (HBR) that falls within section 3 of the Human Biomedical Research Act (HBRA). As part of the review process, the DERC will determine whether the research can be exempted or referred to the NUS Institutional Review Board (NUS-IRB) for review based on the risk to the research participants.
Effective from 8 October 2024, DERCs will be authorized to review faculty research studies that are no more than minimal risk which qualify for Exemption, and are funded by overseas academic research organizations, Singapore Government agencies, or Singapore Government-owned entities, with grant amounts up to $180,000. This is in line with the existing policy for studies funded by MOE, A*STAR and MOH. For faculty research that qualifies for Exemption and is funded by other external sources, such as commercial companies, please submit your applications directly to the NUS-IRB for ethics review. In cases of uncertainty, the Principal Investigator (PI) should submit the application to the NUS-IRB.
This DERC review is further breakdown into 2 categories.
(1) Student Research
PI will need to review the general information and guideline IRB-GUIDE-018 Ethics Review of Student Research V8 2022-10-31 and initiate a new submission via the DERC Review and Approval Management (DREAM) App ( DERC Review and Approval Management App – Power Apps)
PI may need to fill in the form below as applicable and attached to the relevant section of your study submission in DREAM.
PISCF IRB-GUIDE-S03 – V4 2020-05-28 -> need to fill up and submit (If applicable)
*Undergraduate students cannot be PIs.
(2) Faculty Research
PI will need to review the general information and guidelines on
1. IRB-GUIDE-022 Ethics Review of Faculty Research by DERC V3 2022-10-31 -> General information/guidelines
2. SBER Guidelines IRB-GUIDE-S02 – IRB Exemption Form V2.1 2017-08-22 -> General information/guidelineIRB-GUIDE-018
and initiate a new submission via the DERC Review and Approval Management (DREAM) App ( DERC Review and Approval Management App – Power Apps)
IRB Submission workflow
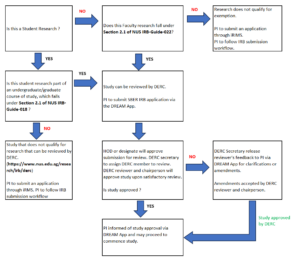
For research that falls under HBR, (2) not of minimal risk, (3) cannot be exempted:
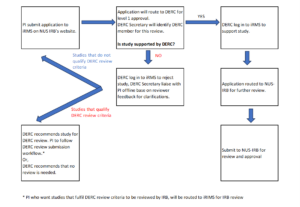
DERC Submission workflow for Social, Behavioral and Educational Research (SBER) that are of minimal risk and can be exempted:
IRB-Guide-022; Version 3 (updated 31 Oct 2022)
Ethics review of Faculty Research that qualify for an IRB exemption by the Department Ethics Review Committee
1. Introduction:
- All faculty research[1] involving humans as research participants must be subject to ethics review by either the NUS Institutional Review Board (NUS-IRB) or the Department.
- From 1 June 2019, faculty research that qualify for an Exemption[2] may be reviewed and approved by a Departmental Ethics Review Committee (DERC).
- However, all human biomedical research as defined by section 3 of the Human Biomedical Research Act (HBRA) 2015 must be referred to the NUS-IRB for review.
The meaning of “human biomedical research” is defined by section 3, HBRA, which provides:
“Meanings of “human biomedical research” and “supervision and control”
3.—(1) In this Act, “human biomedical research” means the research specified in subsection (2) or (3) but subject to subsection (4).
(2) Any research that is intended to study —
(a) the prevention, prognostication, diagnosis or alleviation of any disease, disorder or injury affecting the human body;
(b) the restoration, maintenance or promotion of the aesthetic appearance of human individuals through clinical procedures or techniques; or
(c) the performance or endurance of human individuals,
where the research involves —
(i) subjecting an individual to any intervention (including any wilful act or omission) that has a physical, mental or physiological effect (whether temporary or permanent) on the body of the individual;
(ii) the use of any individually-identifiable human biological material; or
(iii) the use of any individually-identifiable health information.
(3) Any research that involves —
(a) human gametes or human embryos;
(b) cytoplasmic hybrid embryos;
(c) the introduction of any human-animal combination embryo into an animal or a human;
(d) the introduction of human stem cells (including induced pluripotent stem cells) or human neural cells into an animal at any stage of development (including a prenatal animal foetus or animal embryo); or
(e) any entity created as a result of any process referred to in paragraph (c) or (d).
(4) Subsections (2) and (3) do not apply to such research, studies or activities that are specified in the Second Schedule.
(5) For the purposes of this Act, human biomedical research is treated as conducted under the supervision and control of a research institution if the research institution is identified as the research institution for that research and that research has been reviewed by an institutional review board appointed by that research institution.”
- As a general rule, the NUS-IRB does not conduct retrospective reviews for ethics approval of projects as these tend to be problematic.
- The NUS-IRB will issue a statement of concurrence for DERC-approved studies, upon request by the PI for the purpose of publication and/ or release of grant funding.
2. Responsibilities:
- The DERC may review all faculty research involving human subjects when:
- they are construed as “research”;
- the research is excluded from the HBRA;
- the faculty’s research is of minimal risk[3] and qualifies for an Exemption;
- the research does not involve vulnerable populations[4] and/or deception[5] (see IRB-GUIDE-020 on use of deception in research), or sensitive topics;
- the research involves the use of lucky draws in lieu of reimbursement for participation in the study, where the conduct of the lucky draws or research fulfill the criteria as stated in the NUS-IRB’s guidelines for lucky draws; and
- the research does not involve any testing of a medical device or health product as defined in the Health Products Act.
- All research not covered in section 2.1 above will have to be reviewed by the NUS-IRB. The DERC may also refer research studies to the NUS-IRB if they are uncomfortable with undertaking the review. Faculty can also submit their research that qualify for Exemption to the NUS-IRB directly for ethics review.
- From 8 October 2024, faculty research that is externally funded by overseas academic research organizations, Singapore Government agencies, or Statutory Boards, Singapore Government-owned companies up to a grant quantum of $180,000 can be reviewed by DERCs, provided the research also fulfils points 2.1.1 to 2.1.6 of the Guidelines (IRB-Guide-022).
- For all other external grants/ funding, such as commercial companies, please submit the application to the NUS-IRB.
[1] Any systematic investigation with the intention of developing or contributing to generalizable knowledge.
[2] See SBER Guidelines on IRB Exemption Form at http://nus.edu.sg/research/irb/guidelines/sber-guidelines to determine if a study could be exempted.
[3] Risk is considered minimal where the probability and magnitude of harm or discomfort anticipated in the research are not greater, in and of themselves, than those ordinarily encountered in daily life or during the performance of routine physical or psychological examinations or tests.
[4] Refers to subjects who may be unduly coerced or influenced to participate (e.g. children, prisoners, pregnant women, cognitively impaired persons, or educationally disadvantaged persons who require special consideration to protect their welfare.)
[5] Deception occurs when (i) a researcher deliberately gives subjects false information about some aspect of the research; or (ii) knowingly withholds information about the real purpose or nature of the research (i.e. incomplete disclosure or concealment).
IRB-Guide-018; Version 8 (updated 31 Oct 2022)
Ethics review of Student Research Involving Human Research Participants
1. Introduction:
- All student research[1] involving humans as research participants must be subject to ethics review by either the NUS Institutional Review Board (NUS-IRB) or the Department. This is an important learning experience for both undergraduates and graduate students.
- These research projects may be carried out by individual students. The results from such research may or may not be intended for publication or presentation outside the classroom. They include experimental protocols, observational studies, interviews, questionnaires, secondary use of data that are not in the public domain and research involving human remains, tissues, or biological fluids.
- The Departmental review of both undergraduate and graduate student research can be conducted by the Head of Department or his representative or a Departmental Ethics Review Committee (DERC). They will determine whether the research can be exempted or expedited (minimal risk projects) or referred to the NUS-IRB for review based on the risk to the research participants.
- However, all human biomedical research as defined by section 3 of the Human Biomedical Research Act (HBRA) 2015 must be referred to the NUS-IRB for review.
- The meaning of “human biomedical research” is defined by section 3, HBRA, which provides:
“Meanings of “human biomedical research” and “supervision and control”
3.—(1) In this Act, “human biomedical research” means the research specified in subsection (2) or (3) but subject to subsection (4).
(2) Any research that is intended to study —
(a) the prevention, prognostication, diagnosis or alleviation of any disease, disorder or injury affecting the human body;
(b) the restoration, maintenance or promotion of the aesthetic appearance of human individuals through clinical procedures or techniques; or
(c) the performance or endurance of human individuals,
where the research involves —
(i) subjecting an individual to any intervention (including any wilful act or omission) that has a physical, mental or physiological effect (whether temporary or permanent) on the body of the individual;
(ii) the use of any individually-identifiable human biological material; or
(iii) the use of any individually-identifiable health information.
(3) Any research that involves —
(a) human gametes or human embryos;
(b) cytoplasmic hybrid embryos;
(c) the introduction of any human-animal combination embryo into an animal or a human;
(d) the introduction of human stem cells (including induced pluripotent stem cells) or human neural cells into an animal at any stage of development (including a prenatal animal foetus or animal embryo); or
(e) any entity created as a result of any process referred to in paragraph (c) or (d).
(4) Subsections (2) and (3) do not apply to such research, studies or activities that are specified in the Second Schedule.
(5) For the purposes of this Act, human biomedical research is treated as conducted under the supervision and control of a research institution if the research institution is identified as the research institution for that research and that research has been reviewed by an institutional review board appointed by that research institution.”
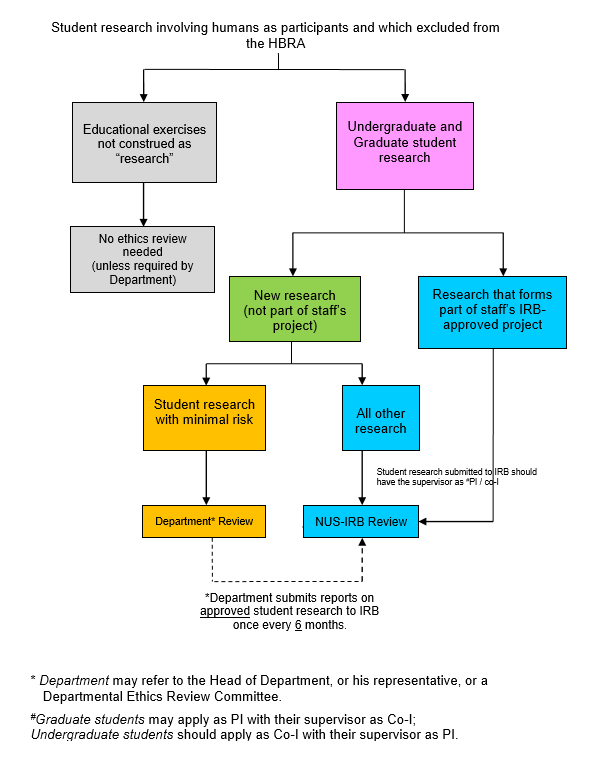
- Educational exercises involving human participants that are not construed as “research” (e.g., class demonstrations and exercises that do not lead to publishable materials) do not require ethics review, unless otherwise required by the Faculty or Department Heads.
- Retrospective reviews for ethics approval of projects tend to be problematic and are not encouraged.
- The NUS-IRB will issue a statement of concurrence for DERC-approved studies, upon request by the PI for the purpose of publication and/or release of grant funding.
2. Responsibilities:
- The Department may review all student research involving human subjects when:
- they are construed as “research”;
- the research is excluded from the HBRA;
- the students’ research is of minimal risk[2];
- the research does not involve vulnerable populations[3] and/or deception[4] (see IRB-GUIDE-020 on use of deception in research);
- the research involves the use of lucky draws in lieu of reimbursement for participation in the study, where the conduct of the lucky draws or research fulfill the criteria as stated in the NUS-IRB’s guidelines for lucky draws.
- the research is not part of a faculty member’s research project already subject to review by NUS-IRB.
- the research does not involve any testing of a medical device or health product as defined in the Health Products Act.
- Student research that are supervised by individuals outside the faculty (including persons not affiliated to the University) may also be reviewed by the Department, unless they are subject to review by the NUS-IRB or by another IRB in compliance with the existing guidelines.
- All research not covered in section 2.1 above will have to be reviewed by the NUS-IRB. The Department may also refer research studies to the NUS-IRB if they are uncomfortable with undertaking the review.
- Research conducted by students that are not part of an undergraduate or graduate course of study must be reviewed by NUS-IRB.
- For applications to the NUS-IRB, undergraduates will have to do so with their supervisors as the main applicants. Graduate students may apply as the “Principal Investigator” with their supervisors as the “Co-investigator”.
Please refer to the flowchart below that illustrates the above.
[1] Any systematic investigation with the intention of developing or contributing to generalizable knowledge.
[2] Risk is considered minimal where the probability and magnitude of harm or discomfort anticipated in the research are not greater, in and of themselves, than those ordinarily encountered in daily life or during the performance of routine physical or psychological examinations or tests.
[3] Refers to subjects who may be unduly coerced or influenced to participate (e.g. children, prisoners, pregnant women, cognitively impaired persons, or educationally disadvantaged persons who require special consideration to protect their welfare.)
[4] Deception occurs when (i) a researcher deliberately gives subjects false information about some aspect of the research; or (ii) knowingly withholds information about the real purpose or nature of the research (i.e. incomplete disclosure or concealment).