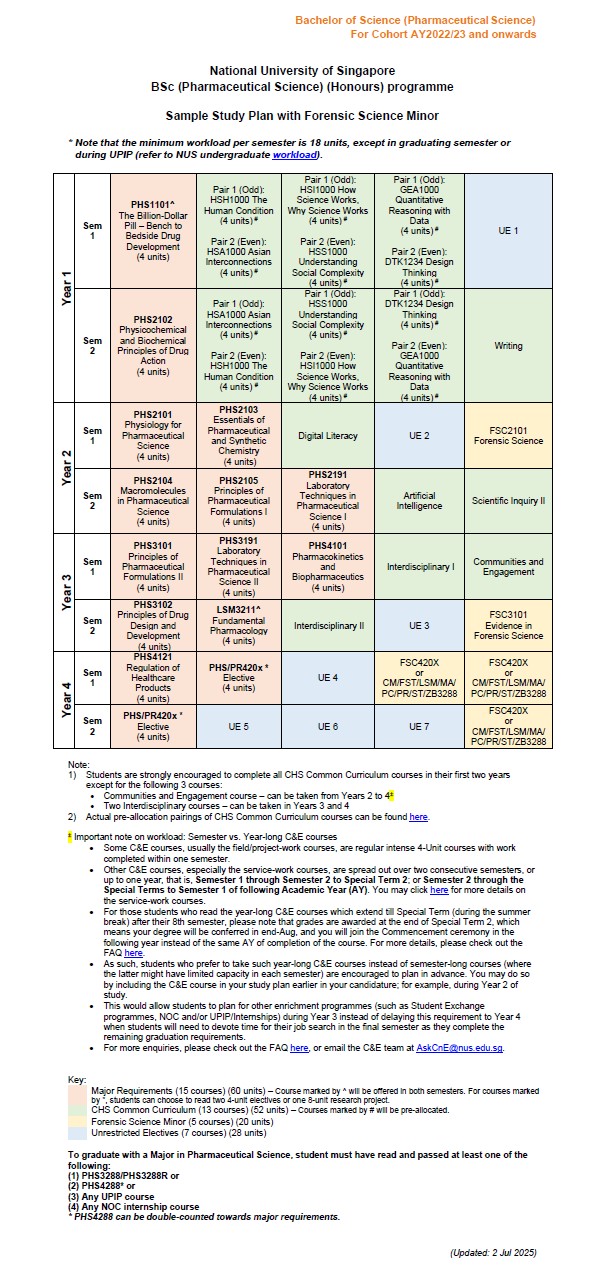
FAQs
PROGRAMME INFORMATION
1. What is Pharmaceutical Science?
Pharmaceutical science belongs to a branch of science that comprises a range of scientific subjects dealing with various aspects of discovery, development, formulation, manufacturing as well as the quality assurance of pharmaceutical substances that are used to manufacture the medicinal products. Therefore, pharmaceutical science forms the foundational scientific basis of the physical, chemical, biological and the biomedical aspects of drug properties and actions.
Some examples of subjects that are classified under pharmaceutical science include medicinal chemistry, pharmaceutics, pharmaceutical technology, pharmaceutical analysis, pharmacokinetics, pharmaceutical biotechnology, pharmacoeconomics and pharmacogenetics. Advancements achieved in pharmaceutical science will impact drug discovery, drug formulation as well as the regulation and practice of pharmacy.
2. What is the role of Pharmaceutical Scientists?
A pharmaceutical scientist is a qualified expert in aspects of the science and technology of medicinal products. This includes, but is not limited to, the discovery, development, manufacture, regulation and utilisation of medical products. Pharmaceutical scientists focus on how medicines work, how safe and effective products are brought to the market, their impact on the body and their effect on the prevention and treatment of disease.
Pharmaceutical scientists are hence equipped with specialised skills for jobs related to research and development, manufacturing, regulatory affairs, medical affairs, clinical trial management, quality control and assurance, sales and marketing, and entrepreneurship.
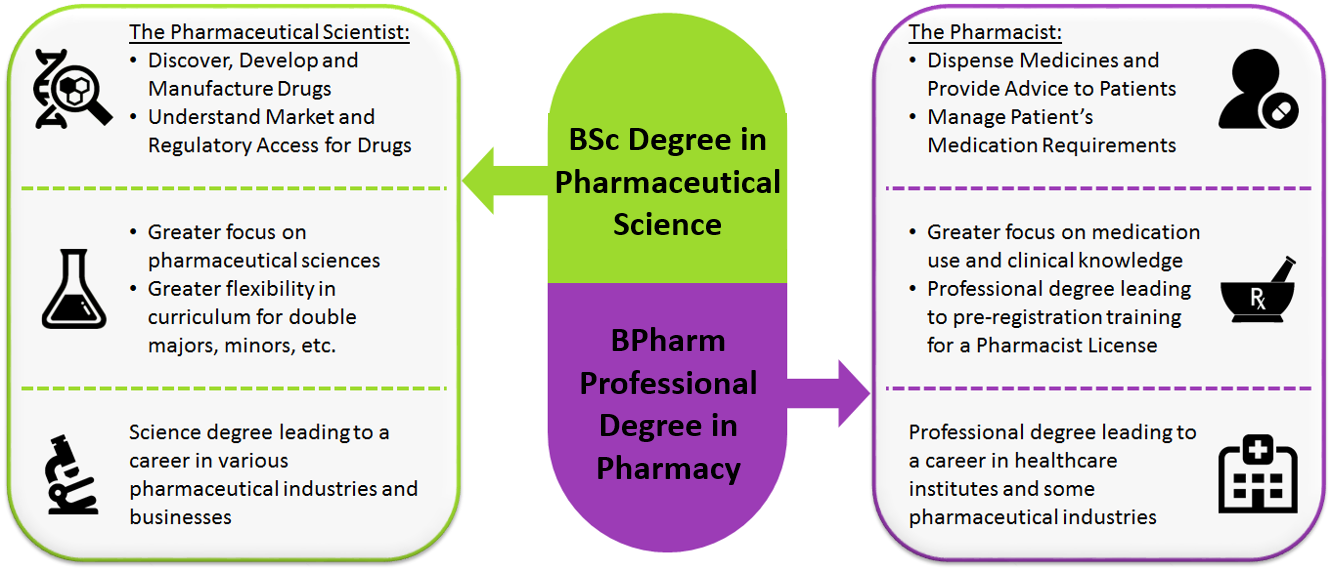
3. What is the difference between Pharmacy and Pharmaceutical Science?
Pharmacy involves the study of drug substances, medicinal products and the use of medicines in patients to achieve optimal therapeutic outcomes. While there are some foundational pharmaceutical sciences covered in the NUS Pharmacy programme, this programme is chiefly clinical in nature and leads to a professional healthcare degree and pharmacist licensing to support the handling and transactions of medications. As licensed holders of medication, pharmacists will be well-trained to advance patient-focused, medicine-centred healthcare practices.
This focus is in contrast to that of pharmaceutical scientists, who work towards the discovery, development, testing, manufacturing and understanding of the market and regulatory access of medicines. The NUS Pharmaceutical Science programme will cover a broad range of these disciplines in greater depth, but will not delve into clinical practice and does not lead to licensing like a pharmacist.
4. What is the programme duration?
The Pharmaceutical Science programme is a four-year degree programme. You are admitted directly into the Pharmaceutical Science programme in the first year of study. At the end of the four years, graduates are conferred the degree of BSc in Pharmaceutical Science, while those who have demonstrated good academic performance over the four years will be awarded the BSc (Hons) in Pharmaceutical Science degree.
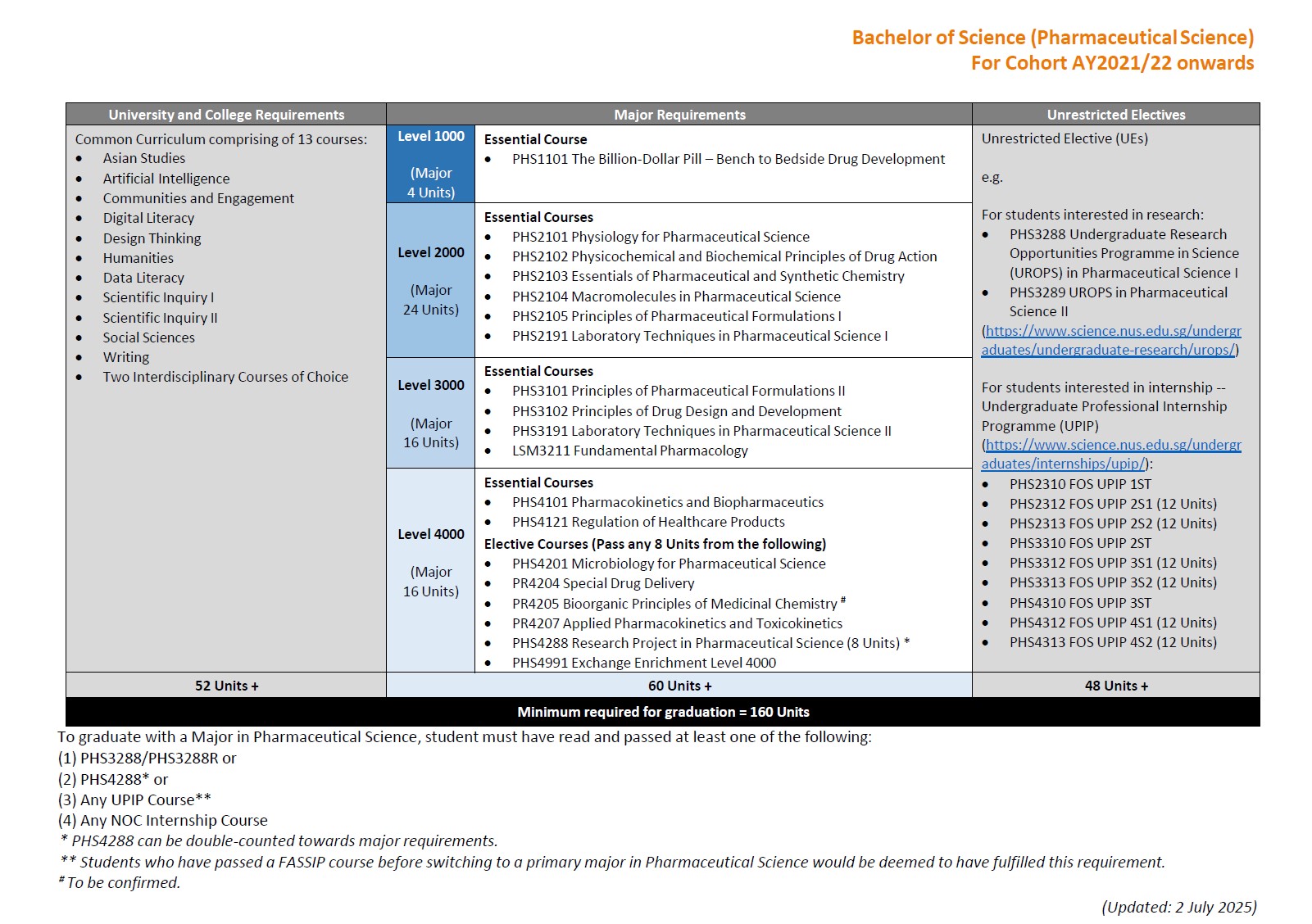
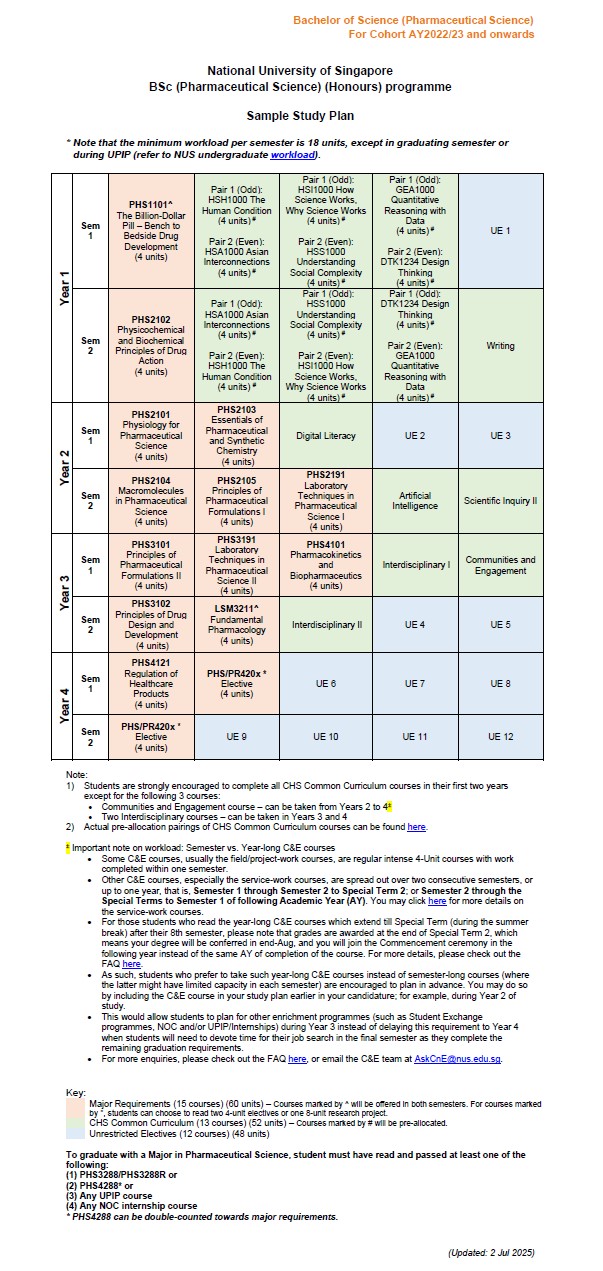
5. What is the overall programme structure like?
The four-year direct honours degree programme will feature a mixture of interactive lectures, tutorials/collaborative learning workshops and practicals, with blended as well as experiential learning. You will undertake core courses in foundational sciences (e.g. physiology, foundations in pharmacological science, analytical/separation science and biopharmaceutics) before covering the full spectrum of pharmaceutical sciences (e.g. drug discovery and development).
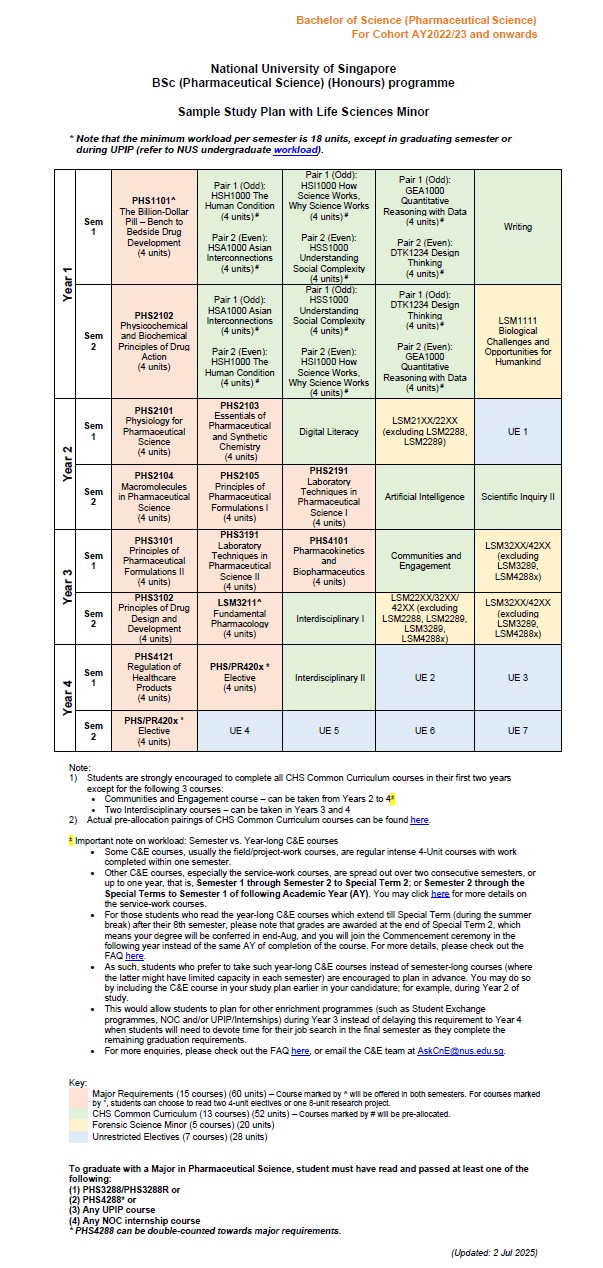
In the 4th year, you are free to choose elective courses in more specialised areas of study (e.g. special drug delivery, applied pharmacokinetics and toxicokinetics). The curriculum also provides flexibility to pursue a broad-based education to explore other interests, such as taking up Double Majors or Minor programmes, e.g. Minor in Forensic Science, Business or Analytical Chemistry. During the four-year programme, you may concurrently participate in other academic programmes offered by the University or Faculty, e.g. NUS College Programme, University Town College Programme, NUS Overseas College Programme, Undergraduate Professional Internship Programme, Undergraduate Research Opportunities Programme in Science, as well as student exchange programmes etc.
APPLICATION/ADMISSION
6. What are the prerequisites for admission into Pharmaceutical Science?
Candidates should have very good passes in Chemistry (H2) and in either Biology (H2) or Physics (H2) or Mathematics (H2) or Further Mathematics (H2) taken at the GCE ‘A’ Level (or equivalent). For information on the Indicative Grade Profile for Pharmaceutical Science Programme (Direct Admission) in Academic Year 2024/2025, please go to https://www.nus.edu.sg/oam/admissions/indicative-grade-profile. For further information, please go to http://www.nus.edu.sg/oam and https://www.askadmissions.nus.edu.sg/ and read the relevant application procedures for respective groups of applicants.
7. Is it possible to enter the Pharmaceutical Science programme without ‘A’ Level Biology?
Yes. ‘A’ Level Biology (H2) (or the equivalent) is not a compulsory subject prerequisite for application to the Pharmaceutical Science programme in NUS. ‘A’ Level Chemistry (H2) (or the equivalent) remains as the only compulsory prerequisite subject for entry to the NUS Pharmaceutical Science programme.
8. How many students are accepted each year?
In Academic Year 2024/2025, we admitted around 70 students into the programme. We anticipate a similar number in the near future.
9. Should I apply for the BSc in Pharmaceutical Science or the Second Major in Pharmaceutical Science or the Minor in Pharmaceutical Science?
The BSc programme (15 Pharmaceutical Science courses) covers a greater depth and breadth of knowledge compared to the Second Major programme (10 Pharmaceutical Science courses) and the Minor programme (5 Pharmaceutical Science courses).
The four-year BSc in Pharmaceutical Science aims to train future pharmaceutical scientists with solid foundations in both theory and hands-on skillsets that are required for entry into the pharmaceutical sector. The programme focuses on the pharmaceutical sciences, culminating in an understanding of drug discovery and development, and appreciation of the regulatory and commercial environment in the pharmaceutical industries. You will be equipped to address complex pharmaceutical problems through integrated knowledge of pharmaceutical chemistry, biology, formulation science, technology and regulatory sciences. There are wide-ranging experiential learning opportunities that enhance readiness for the real world, such as industry internships, research attachments and overseas exchange programmes. Moreover, students from the BSc in Pharmaceutical Science programme will also be members of the NUS Pharmaceutical Society (NUSPS), our student society, where there are opportunities to take part in, or organise, various student-led activities, ranging from freshman orientation projects to welfare events. These experiences will give you a holistic university education with a vibrant university life.
The Second Major in Pharmaceutical Science aims to train Integrators who could connect the dots of the bigger picture, drawing knowledge from the pharmaceutical niche acquired in this second major combined with your primary majors from the sciences, social sciences or humanities, to address real-world issues in the pharmaceutical sector via an interdisciplinary approach.
The Minor in Pharmaceutical Science aims to train Versatilists who appreciate the complexity of the pharmaceutical sector with awareness that complements your primary majors.
OTHER INFORMATION
10. What are the various opportunities that a pharmaceutical science undergraduate can expect, i.e., leadership building, research opportunities etc.?
You will have abundant opportunities to hone your talents and soft skills in NUS Pharmacy and Pharmaceutical Sciences.
NUS Pharmaceutical Society (NUSPS), a student-led organisation, comprises several sub-committees (e.g. media resource team, international relations) that work closely together with the mission to engage and empower the student body. You can be part of NUSPS by serving in the committees and in the process, develop interpersonal, leadership and networking skills, amongst many others.
You can also participate in other special activities/projects e.g. Pharmacy Youth Expedition Project, spend a year at one of the NUS Overseas Colleges, or participate in overseas exchange programmes. These experiences will broaden your horizons.
If you are interested in embarking on a research career, you can participate in the Undergraduate Research Opportunities Programme in Science (UROPS) under close supervision by our experienced department faculty members. These research opportunities are open to you as early as your 2nd year in the Pharmaceutical Science programme.
In your final year, you can undertake a final year research project, under supervision by our experienced department faculty members or industry partners. This will allow you to explore interesting and novel scientific or real-world issues and gain invaluable research experience. You may have the opportunity to showcase and present your research work in local or even international conferences.
CAREER PROSPECTS
11. What are the career prospects for pharmaceutical science graduates?
Graduates will be well-equipped to take up a wide variety of careers in pharmaceutical industries and businesses. Depending on their interests and capabilities, graduates can explore jobs in research and development, manufacturing operations, sales and marketing, regulatory affairs, quality management, pharmaceutical supply chain logistics, and clinical trial management.
By taking up a Second Major in Management, or Minor in Management or Entrepreneurship, we also envision our graduates will acquire grounding in commercial knowledge to be the nation’s next generation of pharmaceutical and healthcare entrepreneurs. The more entrepreneurial graduates have also set up their own business ventures.
12. Will pharmaceutical science graduates be able to switch to a totally different career path should they decide to do so upon graduation?
Yes, graduates from NUS Pharmaceutical Science will be exposed to a rigorous academic programme that prepares an individual for self-directed and lifelong learning to adapt to alternative career paths. The curriculum is designed to produce future-ready, well-rounded graduates with skill sets and knowledge that may also be useful in vocations that are not directly related to pharmaceutical science.
However, the Pharmaceutical Science programme does NOT lead to licensure and you will not be able to register or practise as a pharmacist. Consequently, a small number of industry jobs (e.g. regulatory) may still require employees to hold the pharmacist’s license and these jobs will only be available for licensed pharmacists. If you are interested in pursuing a career as a healthcare professional, you should consider applying for the BPharm programme.